Which Statement Describes a Heterogeneous Catalyst
A chemist dissolves a protein in water in a. Which statement describes a catalyst.
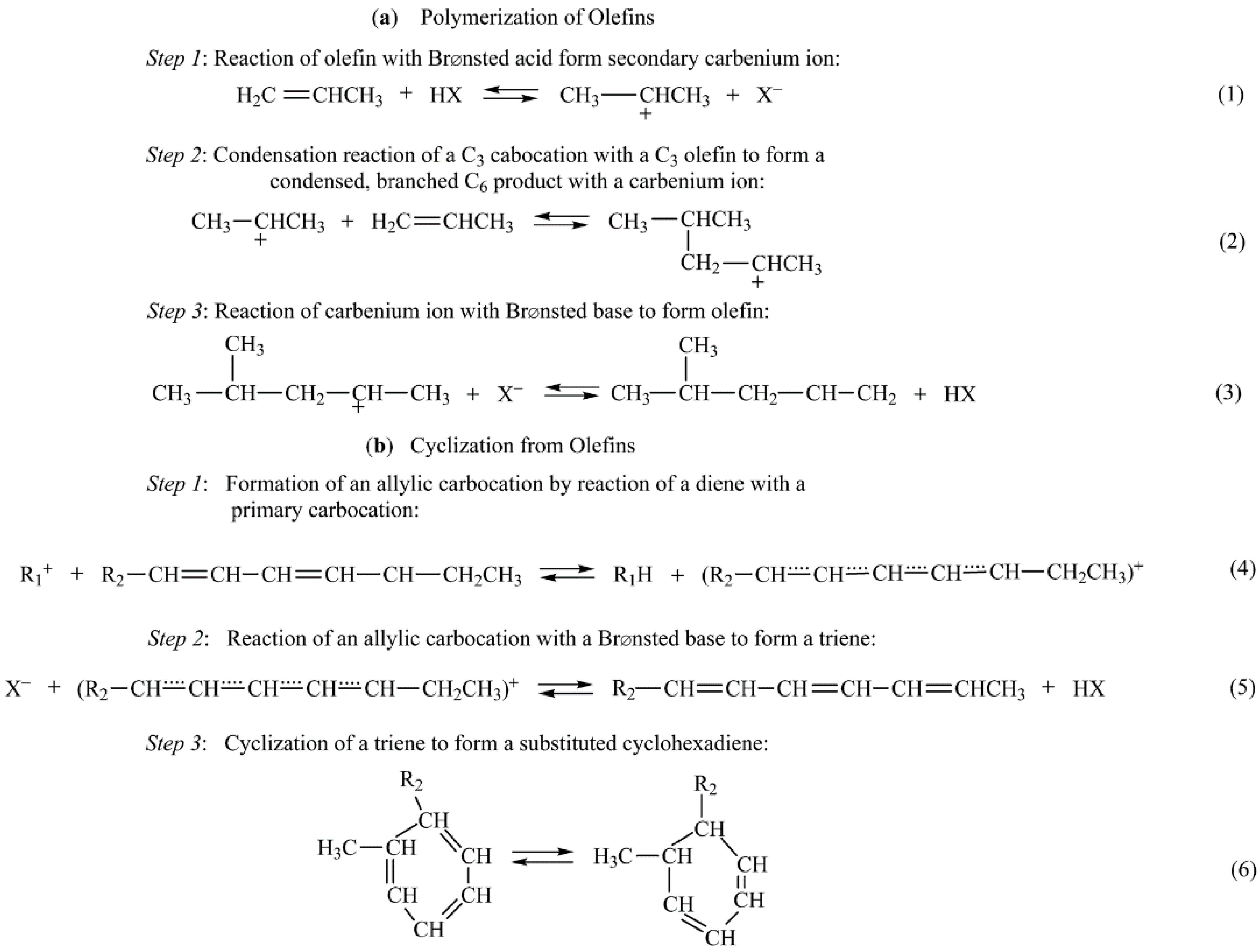
Catalysts Free Full Text Heterogeneous Catalyst Deactivation And Regeneration A Review Html
It is in the same phase as the reactants.

. Which statement describes a homogeneous catalyst. Basically heterogeneous catalysts are generally used in a Solid state and the reactants used are liquids and gasesThis catalyst is very useful as it easily gets separated from the products or the reactants. Heterogeneous catalyst are the catalysis in which catalyst are present in different phase from the reactants and the products.
Nitric acid synthesis Ostwald process NH3 O2 HNO3. Terms in this set 9 Which substance is the catalyst. Homogeneous catalysts are those that occupy the same phase as the reaction mixture typically liquid or gas while heterogeneous catalysts occupy a different phase.
What advantage does a heterogeneous catalyst provide over a homogeneous. It is in a different phase than the reactants. Homogeneous catalyst are the.
Which statement describes a homogeneous catalyst. Which statement describes a heterogeneous catalyst. Statement Describes Heterogeneous Mixtures December 30 2021 However the definition of chemistry includes a wide range of topics that must be understood to gain a mastery of the topic or even take additional courses in chemistry.
It is in a different phase than the reactants. Pepsin is a water-soluble enzyme that helps digest proteins in the human stomach. Generally heterogeneous catalysts are solid compounds that are added to liquid or gas reaction mixtures.
Which statement defines activation energy. Catalysts speed up chemical reactions by increasing surface area. AnswerThe correct answer is option.
Catalyst are those chemical substance which effect the speed of the reaction. It increases the activation energy of a reaction. Catalysts are completely consumed by the reaction.
It lowers the reaction rate. It is in the same phase as the reactants. Ethylene oxide synthesis C2H4 O2 C2H4O.
I think that if you look at the history of the auto-parts industry the fact that it has changed so much over the past two centuries is completely due to its ability to change. Recall that according to collision theory reactant molecules must collide with proper orientation. Homogeneous catalysts are catalytic compounds that are in the same phase as the substances which are going into the reaction phase.
Which characteristic of enzymes allows it to be highly specific in the reactions they catalyze. The reason such catalysts are able to speed up a reaction has to do with collision theory. There are two types of catalyst.
Heteroimplies different as in heterosexual. Heterogeneous catalysts are the products of human ingenuity creativity and innovation. Heterogeneous catalysts are catalytic compounds that are in a different phase from that of the phase of the reaction mixture.
Process Reactants Product s Ammonia synthesis HaberBosch process N2 H2 NH3. It is in the same phase as the reactants. I think at the end of the day they are as much a part of the industry as the competition.
Catalysts appear as reactants in a chemical equation. Hydrogen production by Steam reforming CH4 H2O H2 CO2. Catalysts provide a lower energy pathway for the reaction.
Generally heterogeneous catalysts are solid compounds that are added to liquid or gas reaction mixtures. The statement best describes why industry uses heterogeneous catalysts is-They cause stronger bonds to form between molecules. It is the difference between reactant energy and maximum energy.

Describe How A Heterogeneous Catalyst Works And Explain The Factors That Determine The Effectiveness Of Such A Catalyst Study Com

Which Of The Following Is An Example For Heterogeneous Catalysis Reaction

No comments for "Which Statement Describes a Heterogeneous Catalyst"
Post a Comment